EWSW believes so much in the power of our weight loss program that we have undertaken two separate studies. One is an Institutional Review Board approved Double-Blind, Randomized, Placebo-Controlled, Parallel Trial, as well as, a Prospective, Longitudinal Cohort Study of the EWSW 40-Day Weight Loss Program.
The prospective cohort analysis demonstrated that the Personalized & Supervised “EWSW 40 day weight loss program is highly effective—10% of participants lose an average of over 45 pounds in 40 daysâ€
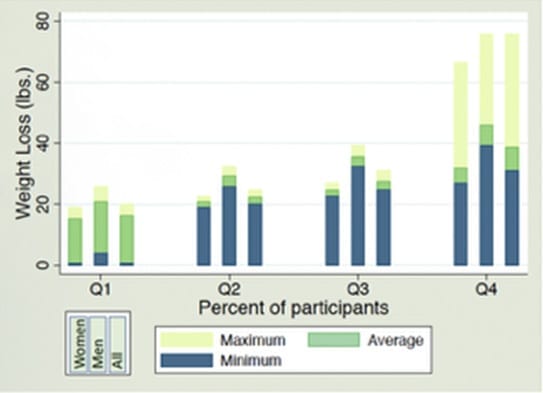
The prospective cohort analysis demonstrated that the top 25% of Personalized & Supervised EWSW 40 day weight loss program participants lost an average of 38.7 pounds.
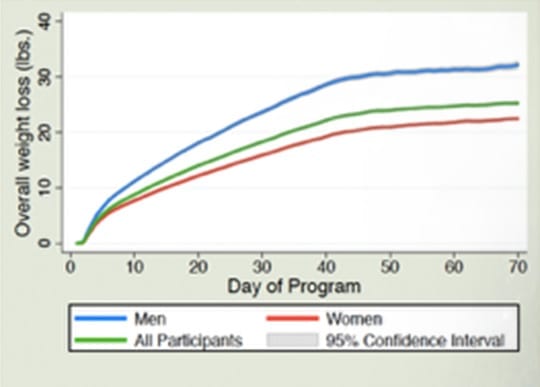
“Overall average weight loss of the 40 day EWSW program was 26.2 pounds—14.3 pounds in the first 20 days on the programâ€*
Both studies demonstrated that the average user not only sustained their weight loss but continued to lose weight after the weight loss phase of the program. The average user in the Prospective study lost over 3 lbs., while the average user of the RCT lost 1.4 lbs. after the weight loss phase of the program.
The IRB approved Randomized Controlled trial demonstrated that participants of the non-personalized & unsupervised 40 day weight loss program safely lost an average of 6.13 pounds in the first 21 days.*
SCHEDULE A CONSULTATION
PROSPECTIVE LONGITUDINAL COHORT ANALYSIS
AVERAGE, MINIMUM, AND MAXIMUM TOTAL WEIGHT LOSS (LBS) BY QUARTILE AND GENDER
TOP 25% OF PARTICIPANTS
Gender | Average Total Weight Loss | Minimum Total Weight Loss | Maximum Total Weight Loss |
Men | 46.0 lbs average men | 39.2 lbs. | 75.8 lbs. |
Women | 31.9 lbs average women | 26.9 lbs. | 66.4 lbs. |
Total (all) | 38.7 lbs average overall | 31.1 lbs. | 75.8 lbs. |
50-75% OF PARTICIPANTS
Gender | Average Total Weight Loss | Minimum Total Weight Loss | Maximum Total Weight Loss |
Men | 35.7 lbs average men | 32.5 lbs. | 39.2 lbs. |
Women | 24.7 lbs average women | 22.7 lbs. | 26.9 lbs. |
Total (all) | 27.6 lbs average overall | 24.8 lbs. | 31.0 lbs. |
25-50% OF PARTICIPANTS
Gender | Average Total Weight Loss | Minimum Total Weight Loss | Maximum Total Weight Loss |
Men | 29.3 lbs average men | 25.8 lbs. | 32.4 lbs. |
Women | 20.9 lbs average women | 19.0 lbs. | 22.7 lbs. |
Total (all) | 22.5 lbs average overall | 20.2 lbs. | 24.8 lbs. |
BOTTOM 25% OF PARTICIPANTS
Gender | Average Total Weight Loss | Minimum Total Weight Loss | Maximum Total Weight Loss |
Men | 21.0 lbs average men | 4.0 lbs. | 25.7 lbs. |
Women | 15.3 lbs average women | 0.6 lbs. | 19.0 lbs. |
Total (all) | 16.3 lbs average overall | 0.6 lbs. | 20.1 lbs. |
The EWSW 40-Day Weight Loss Program also called the EWSW 40-Day Classic program is the name given to the 65+ day program that consists of 6 Phases. EWSW provides personal coaching and other resources over a 65+ day period of which between 40 to 45 days involve low calorie (800 calorie) and other dietary and lifestyle modifications.
Prospective Longitudinal Cohort Case Study Data comes from first-time client submitted data to a third party for tracking of daily weight loss and progress through the EWSW weight loss programs. All results are accurately and transparently presented.
IRB Approved Randomized Controlled Trial was sponsored by EWSW LLC and conducted by an independent contract research organization (Global Clinicals, Inc., Los Angeles, CA).
STUDY COMPARISON
PROSPECTIVE LONGITUDINAL COHORT ANALYSIS | IRB APPROVED RANDOMIZED PLACEBO-CONTROLLED TRIAL |
GOALS | GOALS |
PERSONALIZED | NONPERSONALIZED |
SUPERVIZED |
STUDY COMPARISON
MAXIMUM WEIGHT LOSS ATTAINED OVER 40 DAYS, BY QUARTILE
CUMULATIVE WEIGHT LOSS OVER TIME
IRB APPROVED DOUBLE-BLIND RANDOMIZED PLACEBO-CONTROLLED TRIAL:
Lipid Panel-Change from Baseline Day 45
Lipid Panel | Active Group | Control Group | P-value* |
Cholesterol | -8.33 (20.77) | -1.45 (25.40) | 0.2209 |
Triglycerides | -11.53 (29.81) | 0.39 (38.44) | 0.1528 |
HDL Cholesterol | -0.75 (7.28) | 0.97 (10.31) | 0.4307 |
Cholesterol/HDL | -0.08 (0.79) | -0.05 (0.52) | 0.8627 |
LDL | -5.39 (15.84) | -2.48 (18.89) | 0.4901 |
VLDL | -2.19 (5.99) | 0.06 (7.72) | 0.1778 |
Lipid Panel-Change from Baseline Day 21
Lipid Panel | Active Group | Control Group | P-value* |
Cholesterol | -11.72 (17.47) | -4.36 (23.74) | 0.1450 |
Triglycerides | -4.20 (29.66) | 0.54 (31.96) | 0.5250 |
HDL Cholesterol | -2.61 (6.63) | 0.18 (6.98) | 0.0931 |
Cholesterol/HDL | 0.02 (0.63) | -0.09 (0.48) | 0.4305 |
LDL | -8.28 (14.36) | -4.97 (18.05) | 0.4006 |
VLDL | -0.83 (5.83) | 0.42 (6.02) | 0.3813 |
CONCLUSION
Effectiveness of the Program:Â
Both studies demonstrate that the EWSW 40-Day Weight Loss Program is effective for weight loss.Â
Safety:Â
The RCT trial utilized multiple factors including comprehensive blood work to demonstrate that the EWSW 40-Day weight loss program resulted in safe weight loss.
Sustainability of the Weight Loss:Â
Both the Prospective Longitudinal Trial and the Randomized Controlled Trial demonstrated sustainability of the weight loss with the RCT showing the weight loss continued after the weight loss phase from day 45 to day 112 during which time the calories were substantially raised with the average person losing an additional 1.4 lbs. on average. The Prospective trial demonstrated that during the 21 to 30 days after the weight loss phase “comparing the set point weight to the maximum of the weights reported throughout the entire maintenance period revealed an average continued weight loss of 3.1 lbs. for women and 3.3 lbs. for men. 84.2% of women and 80.0% of men had no weight gain or continued to lose weight compared to their weight set point throughout a one-month period following the last day of the weight reduction phase of the program and after a substantial increase in calories added back to diet. 95.0% of women and 92.1% of men maintained a weight within 2 lbs. of their set point weight.â€
The results of this study indicates that the EWSW weight loss program safely and effectively effectuated weight loss results.Â
EWSW has received STRONGSCIENCE Level 2 certification for efficacy and safety of its weight loss programÂ
SCHEDULE A CONSULTATION
